- Why private practice will survive in the DSO era
- Rehab hospitals with the most, fewest preventable readmissions
- Geisinger names new CFO
- ‘We’re running in when others are running out’: Stability drives record growth for regional Medicare Advantage plans
- ‘We’re running in when others are running out’: Stability drives record growth for regional Medicare Advantage plans
- Freshpaint elevates marketing chief to CEO
- California provider opens teen mental health center
- Catholic Health to outsource Epic support to Nordic
- Idaho GI group cuts appointment wait times with virtual platform
- Longtime Missouri hospital CEO retires
- 6 Texas physicians to pay $5M to settle false claims allegations
- Inside Huntsman’s hybrid model boosting social worker capacity sixfold
- 12 dental technology updates to know
- Imagen Dental Partners adds California practice
- 5 healthcare roles with the most signing bonuses: Indeed
- South Carolina’s largest independent multispecialty group acquired
- Gastroenterology and private equity in 2026: 5 notes
- How Sutter Health is turning AI into a people-first transformation engine
- WHO calls for environmentally friendly, less invasive dental care: 5 notes
- The GI physician shortage by state, by 2036
- GLP-1s tied to higher osteoporosis risk, studies suggest
- ASC vs. HOPD costs for 5 orthopedic procedures
- The Perfect Fit: How Health Systems Are Improving OR Performance with Application-Specific Gloves.
- Federal lawmakers introduce bill to reverse Medicaid cuts, expand Medicare dental coverage: 4 notes
- Maryland awards $1.6M for substance use disorder, peer recovery workforce expansion
- SSM Health adds gastroenterologist
- 3 new ASCs in Florida
- Kaiser Permanente gets green light for 160K-square-foot ASC
- Mount Sinai, Saudi university partner on familial IBD research
- San Diego provider opens 32-bed residential mental health facility
- AI Therapist? It Falls Short, a New Study Warns
- Nevada hospital to downsize, switch to rural emergency status
- Moody’s downgrades Arkansas system’s credit rating
- Mental health providers subject to ban on youth ‘transition’ procedures: Texas attorney general
- Innovate 32 continues growth, adds 2 dental practices in Tennessee
- Indiana hospital transitions revenue cycle operations to Revology
- Mayo Clinic posts 6.8% margin in 2025
- Nearly 20 States Scale Back HIV Medication Programs
- BBQ Sauce Recall Issued Nationwide Due To Incorrect Label
- FDA Recalls More Than 651,000 Jugs of Water Over Sanitation Concerns
- Listen to the Latest ‘KFF Health News Minute’
- Corewell Health posts 1.6% operating margin, grows revenue to $17.6B — 7 things to know
- Hasta los pacientes se sorprenden por los precios que sus aseguradoras están dispuestas a pagar, un costo que al final pagamos todos
- Patients with multiple chronic diseases are a looming threat to health systems' financials: Vizient
- Guardant picks Patrick Dempsey for colorectal cancer blood test awareness
- Breast Cancer Cases, Deaths Expected To Rise Worldwide
- Collagen Supplements Good For Skin, Arthritis, Evidence Review Concludes
- Illicit Adderall Use Places Stress On The Heart, Study Shows
- A-Fib Drug, Diltiazem, Could Interact With Blood Thinners, Increase Risk Of Dangerous Bleeding
- How to Get Ready For Daylight Saving Time
- Effective Sunscreen Protection Can Cost $40 A Year
- Longtime Cigna CEO David Cordani to retire, Brian Evanko tapped as successor
- Acadia, undaunted by recent EU rejection, seeks CHMP re-examination of Rett syndrome med Daybue
- FDA’s CRLs reveal critical errors in AstraZeneca’s Saphnelo data, efficacy doubts for GSK’s Exdensur
- Even Patients Are Shocked by the Prices Their Insurers Will Pay — And It Costs All of Us
- Federal Aid for Lead Cleanup Is Receding. That’s a Problem for Cash-Strapped Cities.
- Readers Lean On Congress To Solve Crises in Research and Rehab
- Disc lays off 20% of employees to steady ship after FDA rejection of rare disease drug
- Novo plugs $500M into Ireland plant to produce Wegovy pill for markets outside US
- Esperion pays $75M-plus to acquire Corstasis and newly approved Enbumyst
- The dental workforce trends that will dominate 2026
- Federal Medicaid cuts threaten dental care access: See the potential impact by state
- Children’s Mercy raises $150M for mental healthcare
- California awards $291M to expand behavioral health housing, services
- OhioHealth builds well-being programs to reshape caregiver culture
- Lawmakers introduce bill to reverse Medicaid cuts, expand Medicare benefits
- New Jersey woman charged with practicing unlicensed dentistry
- 100+ organizations call on CMS to revise 2027 MA rates
- Oklahoma advances interstate compact bill
- UNC Health Appalachian offers psychiatric physician training program
- Colorado Medicaid ABA audit finds $77.8M in improper payments
- UHS to roll out behavioral health revenue cycle AI tools in 2026
- In 1 state, large hospitals dominate 340B's net savings
- CMS to suspend enrollment into Elevance’s Medicare Advantage plans
- Report: Most states investing in value-based care with Rural Health Transformation Program
- U.S. Tops 1,100 Measles Cases This Year as Outbreaks Grow
- FDA To Offer Cash Bonuses for Faster Drug Reviews
- 'One2PrEP': Gilead's 1st Yeztugo DTC ad reimagines hit song to highlight biannual dosing
- GLP-1s support heart attack recovery in rodents by relaxing tight blood vessels
- Former Optum CEO Heather Cianfrocco to depart UnitedHealth Group
- New Drug, Acoziborole, Could Boost Efforts to Wipe Out Sleeping Sickness
- Chocolate Male Supplement Recalled Over Hidden Erectile Dysfunction Drug
- Amid unfolding Middle East war, pharma giants keep close eye on employee safety, supply chains
- CMS set to suspend enrollment in Elevance Health's Medicare Advantage plans
- Providers urge Education Department to reconsider which jobs face stiffer student loan caps
- Kennedy adds 2 new members to CDC’s vaccine panel ahead of delayed meeting
- Kennedy adds 2 new members to CDC’s vaccine panel ahead of delayed meeting
- Urban Traffic Noise Disrupts Sleep, Affects Heart Health After One Night
- Hormone Therapy Might Be Unnecessary For Some Prostate Cancer Patients
- Benzodiazepine Use Down In U.S., But OD Risk Remains, Study Says
- GLP-1 Drugs Might Ease Chronic Migraine, Study Says
- Blood Test Reveals Alcohol-Related Liver Disease
- Telemedicine Visits Cost Five Times Less Than In-Clinic Care
- Families Defend Disability Services Amid Medicaid Cuts
- Medicaid Is Paying for More Dental Care. GOP Cuts Threaten To Reverse the Trend.
- Bavarian Nordic CEO to follow board chair out the door after failed private equity takeover
- Ascendis gains more altitude with FDA approval for dwarfism drug Yuviwel
- CDMO Quotient extends Ipsen supply pact for rare disease drug Sohonos
- Quest Diagnostics launches Google-powered AI chatbot to help patients understand lab results
- Tennr takes aim at phone call bottlenecks as it builds out automation for patient referral process
- DoseSpot, Arrive Health merge to combine prescribing tools with pharmacy, medical benefit data
- Why Digital Tool are Needed to Cope with Increasing Pressures in MedTech Innovation
- Why Digital Tool are Needed to Cope with Increasing Pressures in MedTech Innovation
- Electronics Pollution Pose Added Threat to Endangered Dolphins, Porpoises
- Flea And Tick Pills May Pose Environmental Risks, Study Finds
- ICE, ALS, Addiction Medicine, and Robotic Ultrasounds: Journalists Sound Off on All That and More
- A Canadian Hospital Scoops Up Nurses Who No Longer Feel Safe in Trump’s America
- Statement on the Adoption of Final Rules Under the Holding Foreign Insiders Accountable Act
- Statement on Final Rules for the Holding Foreign Insiders Accountable Act
- State Medicaid budgets to weather $664B reduction through 2034 due to OBBBA: RAND
- Clover Health CEO said company sees opportunity in complex MA environment
- How pharma marketers are capturing the power of podcasts to connect with consumers
- Cigna's Evernorth quietly acquires hospital pharmacy CarepathRx
- Walgreens debuts virtual weight management clinic with access to GLP-1 meds
- New Obamacare Rules Could Raise Deductibles to $31K For Families
- Study Suggests One Common Amino Acid May Affect How Long Men Live
- Merck to wind down Gardasil production at N.C. plant, lay off 150-plus
- Walmart Great Value Cottage Cheese Recalled Over Pasteurization Issue
- Chris Bosh Says He’s 'Lucky To Be Alive' After Sudden Health Scare
- Patrick Kennedy: Collab with MAHA is essential to address mental health crisis
- Lilly debuts Nvidia supercomputer with fanfare and focus on escaping traditional pharma lifecycle
- Alignment CEO John Kao offers measured response to proposed 2027 MA rates
- Sanofi, Genentech, Kedrion back star-studded bleeding disorder awareness campaign
- Op-ed: Our patients deserve better safety reporting. AI could be the answer
- After CHMP nod, Moderna CEO applauds EU's 'rigorous scientific review'
- Blood Test Can Predict Short-Term Survival Among Seniors
- How the Brain Learns to Have Seizures During Sleep
- Why Turning 19 Spikes Medicaid Loss for Millions
- Crash Course Might Speed Brain Stimulation Treatment For Depression, Study Suggests
- Wildfire Smoke Linked To Increase In Violent Assaults
- More Parents Are Refusing A Life-Saving Shot For Their Newborns, Study Finds
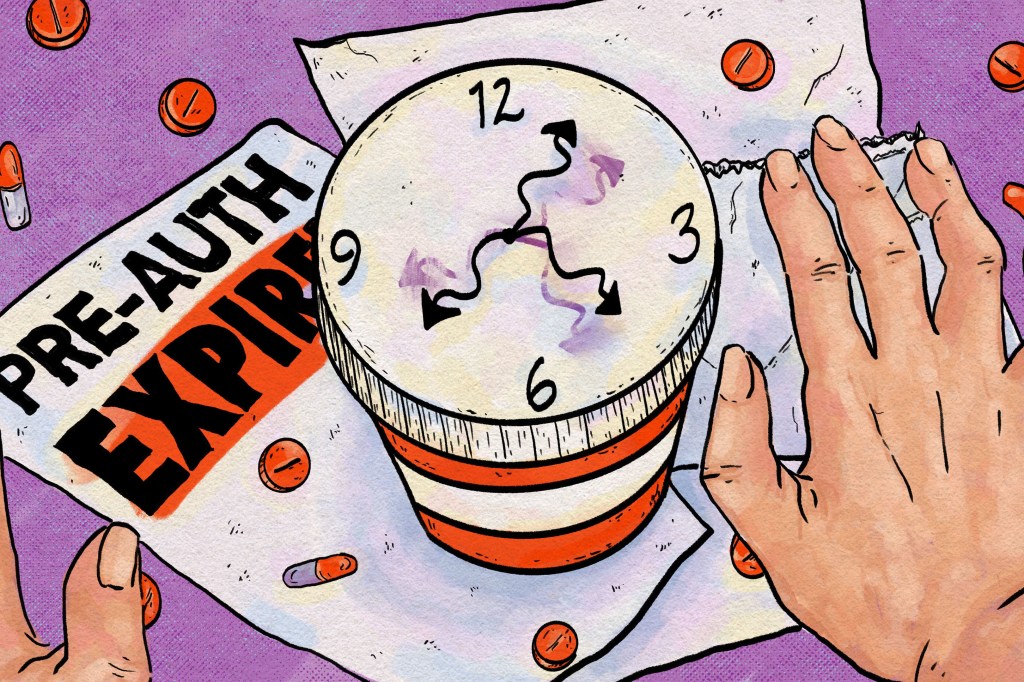
- To Avoid Care Disruptions, Know When the Clock Runs Out on Your Prior Authorization
- Lake Nona Impact Forum: There can't be longevity without tech
- FDA Approval for BIOTRONIK Solia CSP S Pacing Lead For LBBAP
- FDA Approval for BIOTRONIK Solia CSP S Pacing Lead For LBBAP
- Catalyst OrthoScience gets FDA 510(k) Clearance of Archer® Patient-Specific Instrumentation for Shoulder Arthroplasty
- Catalyst OrthoScience gets FDA 510(k) Clearance of Archer® Patient-Specific Instrumentation for Shoulder Arthroplasty
- Smith+Nephew signs distribution agreement with SI-BONE
- Smith+Nephew signs distribution agreement with SI-BONE
- Quantum Surgical Acquires NeuWave Medical, Inc.
- Quantum Surgical Acquires NeuWave Medical, Inc.
- Partnering to Advance Drug Delivery Innovation
- How Pharma is Expanding its Global Footprint to Advance Clinical Research
- Teladoc Health reports slower growth, offers cautious 2026 outlook as it shifts telehealth model
- CFO Mark Kaye to take the helm at Carelon in leadership shake-up at Elevance Health
- Insurance groups say proposed flat Medicare Advantage rates fail to meet the moment
- Stryker launches Synchfix™ EVT, expanding options for flexible syndesmotic fixation
- Stryker launches Synchfix™ EVT, expanding options for flexible syndesmotic fixation
- Democrat-Led States Sue Trump Administration Over Cuts to Childhood Vaccine Schedule
- CDC Vaccine Advisory Panel To Revisit COVID Shot Safety Next Month

Many will welcome less pressure to vaccinate for COVID. This is especially true of people in healthcare.
Importantly, the new policy will likely reveal at last the answers to two hotly-contested claims about COVID vaccines. It may 1) trigger greater COVID severity and case numbers, as vaccine proponents claim; or 2) lead to fewer blood clot, myocarditis, and pregnancy problems, as vaccine opponents claim.
https://www.medpagetoday.com/infectiousdisease/covid19vaccine/117178
Fall COVID Shots Approved, With New Restrictions
— Three vaccines broadly approved for people 65 and older, but with limits for younger groups
August 27, 2025The FDA approved 2025-2026 COVID vaccines from Pfizer (Comirnaty), Moderna (Spikevax, mNexspike), and Novavax (Nuvaxovid), the companies announced on Wednesday. But the agency removed one of the vaccines available for the youngest kids and added limits that will make it difficult for millions of Americans to access the shots.
The mRNA vaccines Comirnaty and Spikevax are approved for all adults 65 and older but limited to people ages 5 to 64 years with at least one condition that puts them at high risk for severe COVID-19. Moderna's Spikevax vaccine is also approved for kids 6 months to 4 years with a high-risk condition. Moderna's lower-dose mRNA vaccine mNexspike and Novavax's protein-based non-mRNA vaccine are approved only for individuals 12 to 64 years with a high-risk condition, and all adults 65 and up.
The three vaccines target the LP.8.1 strain of Omicron, following the advice of the agency's vaccine advisors. Pfizer and Moderna said they expect the vaccines to ship immediately and should be available "in the coming days."
FDA revoked prior emergency use authorizations for the COVID vaccines, which for the Pfizer product previously included a lower-dose shot for children ages 6 months to 4 years.
"The emergency use authorizations for COVID vaccines, once used to justify broad mandates on the general public during the Biden administration, are now rescinded," said HHS Secretary Robert F. Kennedy Jr. in a post on X on Wednesday.
The FDA in May announced their plans to limit COVID vaccines to higher-risk groups, bypassing recommendations from the CDC's Advisory Committee on Immunization Practices (ACIP). Since that time, the FDA has also approved prior versions of Moderna and Novavax vaccines for higher-risk groups.
A recent sweeping review by the Vaccine Integrity Project suggested no new evidence that would prompt the recent changes to fall COVID vaccine recommendations made by HHS. The American Academy of Pediatrics (AAP) also broke with government vaccine recommendations this month, announcing that it strongly recommends COVID-19 shots for children ages 6 months to under 2 years. Shots also are advised for older children if parents want their kids vaccinated, the AAP said.
That differs from the latest guidance under Kennedy, which doesn't recommend the shots for healthy children of any age, but says kids may get the shots in consultation with physicians.
In his post Wednesday, Kennedy said the shots will be "available for all patients who choose them after consulting with their doctors."
Coverage Questions and Access Issues Are Unresolved subhead
Infectious Diseases Society of America (IDSA) President Tina Tan, MD, said the science "strongly supports" vaccination of more than the FDA's newly limited populations.
"By narrowing its approval, FDA has made a decision that completely contradicts the evidence base, severely undermines trust in science-driven policy and dangerously limits vaccine access, removing millions of Americans' choice to be protected and increasing the risk of severe outcomes from COVID," she said in a statement.
Coverage Questions and Access Issues Are Unresolved
In a statement, AAP President Susan J. Kressly, MD, called the new proposal to limit the availability of COVID vaccines in children and young adults "deeply troubling."
"Today's unprecedented action from HHS not only prevents this option for many families, but adds further confusion and stress for parents trying to make the best choices for their children," she said.
AAP "remains focused on increasing access to vaccines for all children, in all communities," said Kressly. "As we enter respiratory virus season, any barrier to COVID-19 vaccination creates a dangerous vulnerability for children and their families. Respiratory illnesses can be especially risky for infants and toddlers, whose airways and lungs are small and still developing."
And Americans are likely to confront a number of logistical hurdles.
Insurers typically base their vaccine coverage decisions on ACIP's recommendations, but some say they will also look to medical professional groups, including the American Medical Association.
Earlier this year, Kennedy ousted the prior ACIP members and replaced its members with a number of doctors and researchers who have repeatedly questioned the safety of commonly used vaccines and ingredients. The panel is expected to meet in September, but no specific date has been set and no agenda released.
Depending on ACIP's advice, Americans under age 65 could be expected to provide documentation of a serious medical condition before they can get a shot. Complicating the rollout is the fact that pharmacists -- who administer most COVID-19 shots -- typically aren't expected to collect that kind of information. And laws governing their ability to administer routine vaccinations vary by state, where pharmacists are licensed.
Many states limit vaccinations by pharmacists to those recommended by ACIP.
"Physicians can still provide COVID vaccines off-label, and IDSA strongly urges doctors to continue recommending and administering vaccination to their patients based on the best available science," Tan said. "However, pharmacists' ability to provide off-label vaccines may be severely constrained, underscoring the vital role of physicians and other clinicians in maintaining access."
Access could also be complicated for healthy adults and children who are interested in getting a shot for extra protection.
If the latest vaccines aren't covered by their insurance, those patients could be required to pay $150 or more out of pocket if they want one.
The Associated Press contributed to this report.
Terrence Rudd is a staff writer at MedPage Today, covering the infectious diseases beat. He has been a medical writer and editor for more than 30 years.
Get MHF Insights
News and tips for your healthcare freedom.
We never spam you. One-step unsubscribe.