- The dental workforce trends that will dominate 2026
- Federal Medicaid cuts threaten dental care access: See the potential impact by state
- Children’s Mercy raises $150M for mental healthcare
- California awards $291M to expand behavioral health housing, services
- Think bigger – Turning AI from Trends to Long-Term Transformation
- UnityPoint Health hospital names market chief nursing officer
- OhioHealth builds well-being programs to reshape caregiver culture
- Washington hospital staff vote ‘no confidence’ in management company
- U of Mississippi Medical Center restores phone lines after cyberattack
- 13 health system IT leadership moves
- How health systems are repositioning informatics
- Mississippi hospital names COO
- Ex-Amazon Health leader joins Spotify founder’s health tech startup
- Know the True Value of Your Lab When an Offer Is on the Table
- U of California offers 32% wage hike in union talks
- UF Health taps new outpatient senior VP
- UAMS names new director of cardiovascular medicine division
- CMS’ add-on billing code boosts specialist pay: Study
- Lawmakers introduce bill to reverse Medicaid cuts, expand Medicare benefits
- Florida medical center to expand with ASC
- New Jersey woman charged with practicing unlicensed dentistry
- CMS extends hospital-at-home waivers for 5 years: What ASCs need to know
- 100+ organizations call on CMS to revise 2027 MA rates
- The retention breakthrough anesthesia needs
- Oklahoma advances interstate compact bill
- Brown University Health names new chief of cardiac surgery
- UNC Health Appalachian offers psychiatric physician training program
- Former PepperPointe Partnerships COO joins DPO
- The Smilist expands into Virginia
- Physician-led orthopedic ASC opens in Florida
- Colorado Medicaid ABA audit finds $77.8M in improper payments
- Georgia opens 30-bed forensic mental health unit to ease jail backlog
- Pennsylvania county cuts ribbon on $19.8M mental health diversion center
- Outpatient cardiology’s CMS whiplash
- 12 new ASCs in February
- UHS to roll out behavioral health revenue cycle AI tools in 2026
- UHS to roll out behavioral health revenue cycle AI tools in 2026
- 15 dentists making headlines
- CMS to suspend enrollment into Elevance’s Medicare Advantage plans
- Report: Most states investing in value-based care with Rural Health Transformation Program
- U.S. Tops 1,100 Measles Cases This Year as Outbreaks Grow
- FDA To Offer Cash Bonuses for Faster Drug Reviews
- 10 providers seeking RCM talent
- PDS Health added de novos across 3 states in February
- Novant posts 4.8% operating margin in 2025
- 'One2PrEP': Gilead's 1st Yeztugo DTC ad reimagines hit song to highlight biannual dosing
- Cleveland Clinic posts $913M operating income, 5% margin — 7 things to know
- Former Optum CEO Heather Cianfrocco to depart UnitedHealth Group
- New Drug, Acoziborole, Could Boost Efforts to Wipe Out Sleeping Sickness
- Chocolate Male Supplement Recalled Over Hidden Erectile Dysfunction Drug
- Clinician Engagement: The Operating Lever Behind Margin and Throughput
- Your Anesthesia Subsidy: Key Questions Every Hospital C-Suite Must Answer
- Amid unfolding Middle East war, pharma giants keep close eye on employee safety, supply chains
- CMS set to suspend enrollment in Elevance Health's Medicare Advantage plans
- Kennedy adds 2 new members to CDC’s vaccine panel ahead of delayed meeting
- Kennedy adds 2 new members to CDC’s vaccine panel ahead of delayed meeting
- Urban Traffic Noise Disrupts Sleep, Affects Heart Health After One Night
- Hormone Therapy Might Be Unnecessary For Some Prostate Cancer Patients
- Benzodiazepine Use Down In U.S., But OD Risk Remains, Study Says
- GLP-1 Drugs Might Ease Chronic Migraine, Study Says
- Blood Test Reveals Alcohol-Related Liver Disease
- Telemedicine Visits Cost Five Times Less Than In-Clinic Care
- Families Defend Disability Services Amid Medicaid Cuts
- Medicaid Is Paying for More Dental Care. GOP Cuts Threaten To Reverse the Trend.
- Bavarian Nordic CEO to follow board chair out the door after failed private equity takeover
- Ascendis gains more altitude with FDA approval for dwarfism drug Yuviwel
- CDMO Quotient extends Ipsen supply pact for rare disease drug Sohonos
- Quest Diagnostics launches Google-powered AI chatbot to help patients understand lab results
- Tennr takes aim at phone call bottlenecks as it builds out automation for patient referral process
- DoseSpot, Arrive Health merge to combine prescribing tools with pharmacy, medical benefit data
- Why Digital Tool are Needed to Cope with Increasing Pressures in MedTech Innovation
- Why Digital Tool are Needed to Cope with Increasing Pressures in MedTech Innovation
- Electronics Pollution Pose Added Threat to Endangered Dolphins, Porpoises
- Flea And Tick Pills May Pose Environmental Risks, Study Finds
- ICE, ALS, Addiction Medicine, and Robotic Ultrasounds: Journalists Sound Off on All That and More
- 11 behavioral health executive moves to know
- 3 behavioral healthcare M&A deals in 2026
- Anthem Blue Cross of California pushes E/M downcoding policy to April
- Iowa dentist surrenders license
- Temple University gets approval for $3.19M rural dental clinic
- A Canadian Hospital Scoops Up Nurses Who No Longer Feel Safe in Trump’s America
- Statement on the Adoption of Final Rules Under the Holding Foreign Insiders Accountable Act
- Statement on Final Rules for the Holding Foreign Insiders Accountable Act
- State Medicaid budgets to weather $664B reduction through 2034 due to OBBBA: RAND
- Clover Health CEO said company sees opportunity in complex MA environment
- How pharma marketers are capturing the power of podcasts to connect with consumers
- Cigna's Evernorth quietly acquires hospital pharmacy CarepathRx
- Walgreens debuts virtual weight management clinic with access to GLP-1 meds
- New Obamacare Rules Could Raise Deductibles to $31K For Families
- Study Suggests One Common Amino Acid May Affect How Long Men Live
- Merck to wind down Gardasil production at N.C. plant, lay off 150-plus
- Walmart Great Value Cottage Cheese Recalled Over Pasteurization Issue
- Chris Bosh Says He’s 'Lucky To Be Alive' After Sudden Health Scare
- Patrick Kennedy: Collab with MAHA is essential to address mental health crisis
- Lilly debuts Nvidia supercomputer with fanfare and focus on escaping traditional pharma lifecycle
- Alignment CEO John Kao offers measured response to proposed 2027 MA rates
- Sanofi, Genentech, Kedrion back star-studded bleeding disorder awareness campaign
- Op-ed: Our patients deserve better safety reporting. AI could be the answer
- After CHMP nod, Moderna CEO applauds EU's 'rigorous scientific review'
- UCB's fast-growing Bimzelx leaps across blockbuster sales threshold as HS momentum builds
- How the Brain Learns to Have Seizures During Sleep
- Blood Test Can Predict Short-Term Survival Among Seniors
- Why Turning 19 Spikes Medicaid Loss for Millions
- Crash Course Might Speed Brain Stimulation Treatment For Depression, Study Suggests
- Wildfire Smoke Linked To Increase In Violent Assaults
- More Parents Are Refusing A Life-Saving Shot For Their Newborns, Study Finds
- He Needs an Expensive Drug. A Copay Card Helped — Until It Didn’t.
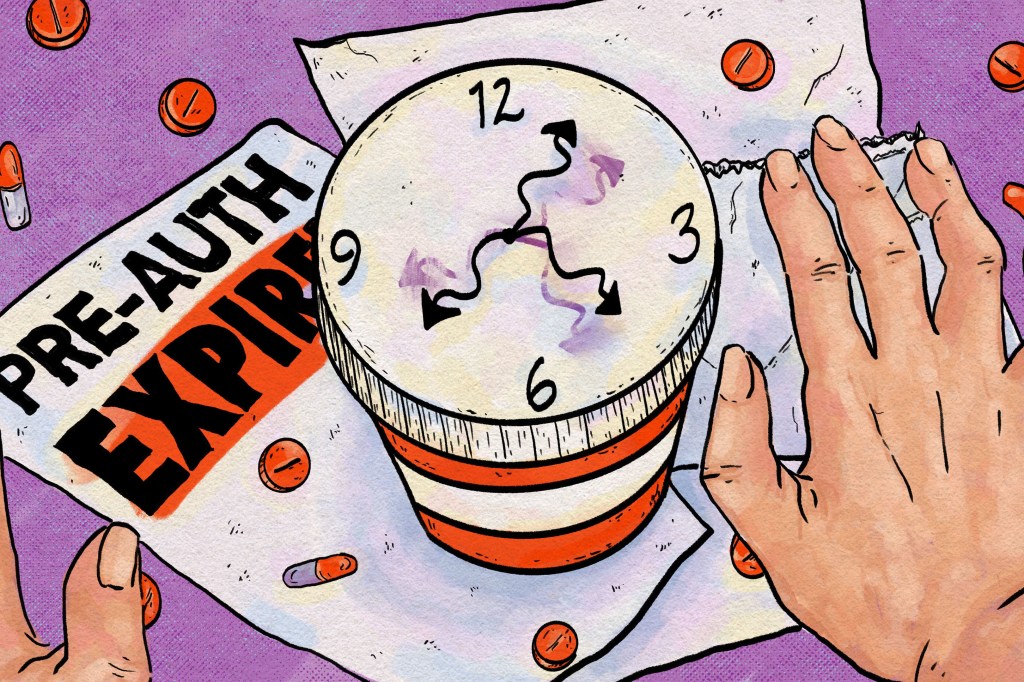
- To Avoid Care Disruptions, Know When the Clock Runs Out on Your Prior Authorization
- As SCOTUS takes on 'skinny label' review, top US lawyer sides with generics maker
- Lake Nona Impact Forum: There can't be longevity without tech
- Fierce Pharma Asia—China deal growth; Daiichi's new CMO; Astellas-Vir bispecific tie-up
- Autoimmune CAR-T: Navigating the FDA’s new regulatory playbook
- FDA Approval for BIOTRONIK Solia CSP S Pacing Lead For LBBAP
- FDA Approval for BIOTRONIK Solia CSP S Pacing Lead For LBBAP
- Catalyst OrthoScience gets FDA 510(k) Clearance of Archer® Patient-Specific Instrumentation for Shoulder Arthroplasty
- Catalyst OrthoScience gets FDA 510(k) Clearance of Archer® Patient-Specific Instrumentation for Shoulder Arthroplasty
- Smith+Nephew signs distribution agreement with SI-BONE
- Smith+Nephew signs distribution agreement with SI-BONE
- Quantum Surgical Acquires NeuWave Medical, Inc.
- Quantum Surgical Acquires NeuWave Medical, Inc.
- How Pharma is Expanding its Global Footprint to Advance Clinical Research
- Partnering to Advance Drug Delivery Innovation
- What the Health? From KFF Health News: What About the State of Health?
- Teladoc Health reports slower growth, offers cautious 2026 outlook as it shifts telehealth model
- CFO Mark Kaye to take the helm at Carelon in leadership shake-up at Elevance Health
- Insurance groups say proposed flat Medicare Advantage rates fail to meet the moment
- Health Gorilla urges court to toss lawsuit filed by Epic, health systems
- Stryker launches Synchfix™ EVT, expanding options for flexible syndesmotic fixation
- Stryker launches Synchfix™ EVT, expanding options for flexible syndesmotic fixation
- Democrat-Led States Sue Trump Administration Over Cuts to Childhood Vaccine Schedule
- CDC Vaccine Advisory Panel To Revisit COVID Shot Safety Next Month
- Frozen Blueberry Recall Issued Across Four States for Listeria
- Boehringer's Hernexeos secures speedy first-line expansion in FDA's 2nd national priority nod
- 'Like talking to a brick wall': Senate hearing takes aim at FDA's rare disease review process
- After delay, CDC vaccine panel sets new dates to discuss long COVID and mRNA shot safety
- Decision Criteria for Technology Commercialization of Medical Devices in 2026
- Decision Criteria for Technology Commercialization of Medical Devices in 2026
- Cientos de enfermeros estadounidenses dejan atrás el Estados Unidos de Trump y eligen trabajar en Canadá
- Continuous Cardiac Monitoring: Redefining the “End” of a Clinical Study?
- Continuous Cardiac Monitoring: Redefining the “End” of a Clinical Study?
- Could Drone-Delivered Defibrillators Save Lives?
- Inflammation Linked To Brain Damage, Memory Problems Among Football Players
- Early Birds, Active Folks Less Likely To Develop ALS
- Disasters Can Affect Mental Health A Decade Later, Review Finds
- AI Chatbots Can Contribute To Worsening Mental Illness, Study Finds
- Newborns Exposed to More ‘Forever Chemicals’ Than Once Thought
- Study Highlights Unique Parenting Struggles of Younger Patients With Heart Disease
- Altman-backed startup Verifiable rolls out AI agent to automate credentialing
- ‘You Aren’t Trapped’: Hundreds of US Nurses Choose Canada Over Trump’s America
- ‘Kind of Morbid’: Health Premiums Threaten Their Nest Egg. A Terminal Diagnosis May Spare It.

No more "one size fits all" vaccine guidances from the CDC's Advisory Committee on Immunization Practices (ACIP):
https://thehill.com/policy/healthcare/5512906-covid-19-vaccine-guidance-update/
CDC vaccine panel votes to change COVID-19 vaccine guidance
By Joseph Choi - September 19, 2025A federal vaccine advisory panel voted on Friday to recommend people talk with a clinician before getting a Covid vaccine, while voting against a motion to require prescriptions for the shot.
All 12 members of the Advisory Committee on Immunization Practices (ACIP) for the Centers for Disease Control and Prevention (CDC) voted unanimously to update COVID-19 guidance so coronavirus vaccinations for all people should be based on “individual-based decision making.”
For people between six months and 64 years old, the recommendation advised that vaccinations be based on individual-based decisionmaking along “with an emphasis that the risk-benefit of vaccination is most favorable for individuals who are at an increased risk for severe COVID-19 disease and lowest for individuals who are not at an increased risk, according to the CDC list of COVID-19 risk factors.”
ACIP Chair Martin Kulldorff said it was his understanding that this recommendation they voted on would mean that SARS-CoV-2 vaccines would still be covered by insurance. Insurers look to the board’s recommendations to inform their coverage.
The ACIP voted against a motion which recommended that states and local jurisdictions require prescriptions for COVID-19 vaccines. The panel does not have the purview over whether to require prescriptions. States and local jurisdictions make those rules, not the CDC.
The vote was split evenly 6-6. With Kulldorff voting ‘no’ to break the tie, the motion failed.
The questions they were to vote on were not publicly disclosed until the very end of the meeting.
ACIP member Retsef Levi, professor of operations management at the Massachusetts Institute of Technology’s Sloan School of Management, led the panel’s discussion on the COVID-19 vaccines.
Levi, a known COVID-19 vaccine opponent and skeptic, was selected to lead the CDC’s COVID-19 working group in August. During the pandemic, Levi called for all COVID-19 programs to be stopped immediately, claiming there was no proof of efficacy and that the vaccines were behind the deaths of children and young people.
Levi presented four questions for the committee to vote on: to recommend the CDC promote six risks and uncertainties he cited in his presentation on Friday; to recommend requiring prescriptions for COVID-19 vaccines; that patients should be informed of the risks of COVID-19 and its vaccination before receiving the shot; and to update the current guidance so that coronavirus vaccinations for all people should be based on “individual-based decision making.”
All questions except for the one recommending a prescription for COVID-19 vaccine were passed by the committee.
Members of the working group that Levi leads gave a presentation strongly supporting the continued availability of COVID-19 vaccines, especially for pregnant women, children and seniors.
“In summary, Covid-19 vaccination matters for pregnant women, pediatric patients — especially those less than two years of age — people 65 years and older, those of any age with a weakened immune system or chronic medical conditions and anyone who feels they want protection for themselves or their families,” said Henry Bernstein, a member of the COVID-19 working group, on behalf of himself and two other members of the working group who he described as being in the “minority.”
During the committee’s discussion on Friday, ACIP members spent time speculating on whether the COVID-19 vaccine rewrote human DNA after being administered, whether it could cause lung cancer; of it could cause birth defects.
The debate over requiring a prescription, though outside the powers of the committee, was prolonged as several members strongly believed that requiring a prescription was creating a barrier to access. Members noted that people who are uninsured or underinsured don’t have the ability to easily go to a healthcare provider to receive a prescription.
Prescriptions aren’t normally required for seasonal vaccines like the flu shot and COVID-19, being available to the demographics for whom they are recommended. Still, Levi argued that COVID-19 vaccines were being treated essentially like over-the-counter drugs, adding that he believed they offered “questionable benefits for a lot of people.”
Fellow ACIP member Hillary Blackburn, pharmacist and director of medication access and affordability at Ascension Rx, who was appointed just this week, spoke out strongly against a prescription requirement.
“Well historically, at least 19 states have required pharmacist authorization to be tied to ACIP recommendations,” Blackburn noted. “And so, a lot of the states have been making their own recommendations to help clarify some of the confusion, which has been limiting some of the access, as we’ve seen with CVS and Walgreens pulling their COVID-19 vaccines for the season.”
Kelly Goode, president of the American Pharmacists Association, added on to this argument, telling the committee, “Pharmacists are the most successful healthcare providers who embrace the relationships with patients, and are well equipped to determine risk based on medications and health histories.”
“Furthermore, pharmacists are the healthcare providers who have the most experience with covid 19 vaccines. Claims data show that 90 percent of COVID-19 vaccines have been given in pharmacies,” she added.
ACIP voted to postpone a decision on the Hepatitis B vaccine for infants and very young children. This has become an issue in the case of Dr. Susan Monarez:
CDC vaccine advisers delay controversial vote on hepatitis B vaccine
By Nathaniel Weixel - September 19, 2025Key vaccine advisers to the Centers for Disease Control and Prevention voted 11-1 Friday morning to delay a recommendation on changes to the hepatitis B vaccine administered to newborns, a surprise development greeted with relief by infectious disease experts.
The vote came after a lengthy and tense discussion Thursday about the necessity of giving newborn babies the vaccine, rather than waiting until they are at least a month old.
Members of the Advisory Committee on Immunization Practices (ACIP) had been considering eliminating the current recommendation that all newborns receive the vaccine, which has been credited with a dramatic decrease in the infection rate over the past 30 years.
Several liaison members of the panel questioned the need for a policy change, given that there was no new evidence showing that there was a problem, or evidence to support delaying the vaccine by a month.
Cody Meissner, a member of the committee, also warned against changing the recommendation during the discussion Thursday.
“We will increase the risk of harm based on no evidence of benefit, because there will be fewer children who will get the full hepatitis B vaccine series,” Meissner said.
The vote was previously scheduled to take place Thursday but was pushed back to Friday.
On Friday morning, ACIP members decided there was too much confusion in the wording of their recommendation.
“I believe that there’s enough ambiguity here and enough remaining discussion about safety, effectiveness, and timing that I believe that a vote today is premature,” panel member Robert Malone said.
“I applaud the committee for getting this one vote right by tabling to allow it to have further discussion,” said Jason Goldman, a liaison representative and president of the American College of Physicians.
Separately Friday, the panel decided to vote again on whether the combined measles, mumps, rubella and varicella (MMRV) vaccine should be covered under the Vaccines for Children (VFC) program, which provides low-cost or free vaccines for about half of the children in the country who are uninsured or on Medicaid.
ACIP members voted Thursday to recommend against using the combined vaccine for kids under 4 years old. But in a confusing addition, they then voted against aligning their recommendation with the VFC program.
The new vote Friday brought the VFC program in line with that new recommendation, meaning the combination shot will no longer be covered. Separate MMR and varicella shots will be.
“Please provide to the public so they can have trust, faith and confidence in vaccination as to what process we are going to be using to properly vet and discuss all future vaccines,” Goldman said. “That is why the evidence to recommend framework was created so we would have the standard to be able to vet all vaccines, but that is not being used.”
ACIP voted unanimously to delay the administration of the vaccine for measles, mumps, rubella and varicella (chickenpox), commonly called the MMRV:
https://thehill.com/policy/healthcare/5511387-mmrv-vaccine-delay-acip/
CDC panel votes to push back MMRV vaccine recommendation to 4 years old
By Joseph Choi - September 18, 2025The vaccine advisory panel for the Centers for Disease Control and Prevention (CDC) voted Thursday in favor of delaying the administration of the vaccine for measles, mumps, rubella and varicella (chickenpox), commonly called the MMRV.
The CDC’s Advisory Committee on Immunization Practices (ACIP) is scheduled to vote on three questions during Thursday’s meeting. Five of the members were appointed to the committee just this week.
First, the panel was asked to consider whether the MMRV vaccine should not be recommended for children younger than 4. The panel voted 8-3 to approve the change, with one member abstaining.
This vote would have meant that children who receive their vaccinations through the Vaccines For Children federal program will not be able to receive the MMRV shot until they’re 4 years old. The committee voted, however, in a follow-up motion against aligning the VFC with the recommendation, changing nothing in terms of what the program covers for the time being.
Children can normally get the MMRV vaccine beginning at 12 months of age.
ACIP members Hillary Blackburn, Cody Meissner and Joseph Hibbeln were the three members to vote no on the recommendation.
The panel decided to delay the votes on hepatitis B vaccine guidance until Friday, when they will also vote on COVID-19 vaccine guidance.
The meeting Thursday was tense, with panel members very aware of the heightened attention on their vote following the firing and resignation of top CDC officials and the growing scrutiny of Health and Human Services Secretary Robert F. Kennedy Jr. on Capitol Hill.
“We are currently experiencing heated controversies about vaccines. And a key question is, who can you trust? Here’s my advice, when there are different scientific views, only trust scientists who are willing to engage with and publicly debate the scientists with other views,” ACIP Chair Martin Kulldorff said at the start of the meeting.
The core argument against allowing MMRV vaccinations under the age of 4 appeared to be the slightly increased risk of febrile seizures linked to the injections. Febrile seizures are caused by fevers of all types.
Febrile seizures are common and generally don’t cause any long-lasting effects, though members of the panel noted they can be traumatic for families to experience. The risk is lower when vaccines are administered when a child is older.
Kulldorff argued the risk of seizures could scare parents away from getting the MMRV vaccination.
Members of the committee who opposed the motion argued that changing the recommendation took away parents’ right to choose when and how to vaccinate their children.
Jason Goldman, president of the American College of Physicians, lambasted the motion as lacking supporting evidence and causing more confusion than benefits.
“When you make this recommendation, you now give license to insurance companies and the Vaccine For Children Program not to cover this vaccine. And finally, you are taking away the choice of parents to have informed consent and discussion with their physician on what they want to do for the health and benefit of their children,” Goldman told the committee during public comments.
Others warned this motion will cause a drop in vaccination rates.
“The disadvantage of giving two doses — or, as was suggested, separating the two doses — is that we know compliance falls. And the advantage of combination vaccines is that children and adults are more likely to complete the vaccine requirements if it’s given as a single dose,” Meissner said.
According to Andy Pavia, professor and pediatric infectious disease expert at the University of Utah, the immunization practices considered Thursday are “settled science.”
“This new handpicked ACIP has chosen to address issues that were relatively settled science for which there’s no new information that really suggests a need to do a detailed review,” Pavia said shortly before the vote. “And has already proposed votes that would change things even before hearing the data, and it suggests a great deal of prejudgment of the issues.”
Former CDC Chief Medical Officer Debra Houry told the Senate Committee on Health, Education, Labor, and Pensions the day before the meeting that the agenda and questions discussed by the ACIP were developed almost exclusively by political appointees, with little input from scientific staffers from her agency.
According to Houry, CDC scientists had no input on the first questions the committee voted for Thursday, only being asked for contributions on the COVID-19 vaccine vote scheduled for Friday.
The public health associations which sued over the Advisory Committee on Immunization Practices (ACIP) COVID-19 vaccine guidance have filed their fourth amendment to cover just about every subsequent CDC vaccine guidance:
Public health groups sue over CDC vaccine recommendations
By Joseph Choi - January 20, 2026A coalition of medical groups have expanded an ongoing lawsuit against Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. to now challenge and reverse the reductions made to the childhood vaccine schedule.
A group of plaintiffs including the American Academy of Pediatrics (AAP), the American College of Physicians (ACP) and the American Public Health Association (APHA) and others sued HHS under Kennedy in July 2025 to undo updated COVID-19 vaccine guidance that no longer included pregnant women and healthy children in the recommended demographics.
In its fourth amended complaint, the lawsuit now additionally challenges the decision by the Centers for Disease Control and Prevention (CDC) earlier this month to reduce the number of recommended childhood vaccines from 17 to 11, bringing the country in line with that of Denmark, a nation Kennedy frequently points to when expounding on vaccine guidance.
The plaintiffs called this decision the “most egregious, reckless, and dangerous of the actions Defendants have taken to date” in their amended complaint filed on Monday. They further called for an upcoming meeting of the CDC’s Advisory Committee on Immunization Practices to be blocked from being held.
Kennedy fired the entire ACIP last year and remade it with many appointees sympathetic to his views, including many known vaccine critics and skeptics.
The plaintiffs argued the current membership of the committee violates federal law that requires committees to be “fairly balanced” and not be “inappropriately influenced.”
The plaintiffs had signaled its intention to sue over the reduction in childhood vaccines soon after the decision was announced.
“Court intervention is now essential to prevent further harm, protect evidence-based recommendations, and ensure that critical decisions affecting children’s health are made transparently and guided by evidence, not ideology,” Richard Hughes IV, attorney for the plaintiffs said in a statement.
“We are confident that we will demonstrate for the court that this administration has acted arbitrarily and capriciously in revisions to the childhood immunization schedule and, furthermore, that the current ACIP will continue this destructive pattern if allowed to continue meeting,” added Hughes.
An HHS spokesperson called the lawsuit “a baseless attempt to litigate for the interests of the organization’s top corporate donors, which make virtually every vaccine across the CDC immunization schedules.”
“AAP is angry that CDC eliminated corporate influence in vaccine recommendations by reconstituting the Advisory Committee on Immunization Practices with leading physicians and public health experts and by accepting recommendations from a comprehensive scientific review of U.S. childhood immunization practices conducted under President Trump’s order to examine international best practices in peer developed countries,” they added.
Get MHF Insights
News and tips for your healthcare freedom.
We never spam you. One-step unsubscribe.